Sabtu, 15 Mei 2010
How to Make Colloid
Types of Colloid
Stability of Colloid
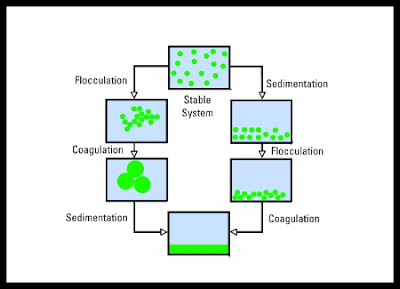
- An initially formed aggregate is called a FLOC and its formation FLOCCULATION - this process is reversible (DEFLOCCULATION)
- If the aggregate changes to a much denser form it is said to undergo COAGULATION - this process is irreversible
Jumat, 14 Mei 2010
Classification of Colloid

There are eight types of Colloidal Mixture, they are Foam, Solid Foam, Liquid Aerosol, Emulsion, Gel, Solid Aerosol, Sol, and Solid Sol. They are different from one another and is distinguished by the continuos medium and dispersed phase that made them. From the table prepared above, we can see the types of colloids and also it's examples. Colloids can't be made from gas continuos medium and gas dispersed medium since all gases are miscible, it is the property of some substances to mix in all proportions forming a homogenous solution and not colloid as a colloid mixture is actually a heterogenous one.
Selasa, 11 Mei 2010
Property of Colloid
There are many property of colloid, each had their own characteristic and definition. from this entry, we will explain to you one by one.
~Tyndall effect: it is the scattering of light trough colloid. it was first explained by the British Physicist, John Tyndall. His theory suggest that if a beam of light were to pass trough a colloid, it would scattered.
~Brownian movement: there are a continuos collision between the particles of colloid and the molecules of dispersion and this will result a random, zig zag movement to the colloidal particle. this movement is called Brownian movement.
~Electrophoresis: it is the movement of Colloidal particle on the influence of an electric field. If an electric field is applied across a colloidal solution, the particles of that solution moves to the opposite charged electrode so they could get neutralized.
~Adsorption: it is the absorbing activity in a colloid mixture which result the absorbing of colloidal particles to a surface.
~etc